
How to Taper Pristiq is now available on Amazon.com Click here
You can also read How to Taper Pristiq below for free on this page.
How to Taper Pristiq
2025 Edition
There is Hope. There is a Solution.
__________
By James Harper
All rights reserved. No part of this book may be reproduced or transmitted in any form or by any means without written permission from the author.
ISBN: 9798282337365
Imprint: Independently published
Printed in USA
Dedication
To my mother, I thank you from the bottom of my heart.
As a child you gave me a safe space to just be a child and allowed me to stumble and learn from my mistakes. As an adult you encouraged me to keep looking for new answers.
With your passing you have helped me learn value in each moment. Thank you and you are missed.
To all of you that have helped along the way, your assistance has been invaluable.
Author contact: Jim@theroadback.org
Table of Contents
Introduction …………………………………. 5
Chapter 1 Tapering Pristiq ……………… 15
Chapter 2 Withdrawal Symptoms ……. 19
Chapter 3 Taper Using Supplements … 51
Chapter 4 Journal ………………………… 57
Chapter 5 Graph ………………………….. 59
Chapter 6 General Information ………. 71
About The Author ………………………… 77
Introduction
Pristiq is new on the market, compared to the standard antidepressants like Prozac, Zoloft and Lexapro, but after 26 years of research, assisting tens of thousands of people to get off their psychotropic medication, this book is the final and closing chapter of the development of How to Taper Pristiq. Not to say there will not be future advancements in a Pristiq withdrawal program, but the foundation needed for a Pristiq withdrawal program and health program is now firmly in place. The success rate of people now using this program is higher than ever imagined, the basic causes of Pristiq withdrawal symptoms and the human health decline have now been discovered, and the solution is backed by scientific evidence.
This Pristiq withdrawal book is written mainly for the patient, or individual wanting to get off Pristiq or to simply rebuild or maintain good health after taking Pristiq.
If you have tried to withdrawal off Pristiq in the past and suffered, have found this book in the middle of your Pristiq withdrawal or quit Pristiq cold turkey, you may have a little more work to do than most, but the success will still be there. With the advancements in How to Taper Pristiq, your path to recovery and achieving well-being—perhaps for the first time in years—can now be attained within a relatively short period.
If you are seeking to improve your overall health, there is usually no need to have patience when you use the program, it works rather quickly. Many of us are just accustomed to feeling how we feel, and we may have lost track of how good we can feel when the body and mind work as a unique team. By doing a few basic things, we can reverse much of what is happening inside of our body and that reversal usually equates to a better attitude in life and a major quality of life improvement.
I want to acknowledge the many people, from the four corners of Earth and all walks of life, who have successfully changed their life while using this program. Their perseverance and feedback have helped advance this program to today’s high degree of success.
And I applaud you, opening this book for the first time, for your courage and resolve to change your life and get yourself back as your reward.
I understand the apprehension you may feel about deciding to come off Pristiq, especially if you have tried to do so before and failed, or if you have heard horror stories of others who have tried to come off Pristiq.
Further, I understand the questions you might be asking at this point:
- Will I experience mental or physical pain while on this program?
- Will I have other side effects while on this program?
- Will the Pristiq side effects get worse before they get better?
- Will my depression get worse during this program?
- Will my anxiety levels increase?
You may have many other questions in addition to those above, but most importantly you should know that How to Taper Pristiq can be virtually side effect free. The testament to this, as you will see throughout the book, is that people just like you start to feel better, mentally and physically, sometimes from day one.
You have two options when using this program. You can use the supplements that are recommended or just reduce the Pristiq slowly. Jim Harper strongly urges you to include the supplements during the taper.
Why supplements? In 1999, when this withdrawal method was first introduced Jim Harper did not recommend supplements. This was when the reduction of 10% of the original dosage was used for antidepressants, antipsychotics and ADHD medication and a 5% taper if reducing a benzodiazepine. You will see these reduction amounts as the standard now for every drug withdrawal program. Jim also introduced the slow and gradual method for tapering.
During 1999 and 2000, over 1 million people read Jim’s website, and hundreds of thousands used his tapering approach. The results led Jim to believe there had to be a much better way to taper these drugs and began looking at specific supplements. With Pristiq prescriptions written quite often in those days, a high percentage of people Jim assisted off their medication were taking Pristiq.
What were the results that made Jim look for other solutions?
Fifty percent of the people could taper off their medication by tapering gradually and slowly and by using the 10% or 5% reduction method. The medical community viewed these results as a major breakthrough in tapering a psychotropic medication. The underlying story told a different story altogether.
Of the 50% that made it off their medication, 5% made it off their drug with ease and were able to remain off the drug. However, the other 95% suffered their way through the taper and eventually went back on the medication to get relief from the lingering withdrawal side effects.
Jim Harper would not accept these results as a success.
In 2000, some promises of a better taper method were seen using a whey protein powder that is manufactured to increase a substance called glutathione. Glutathione is the body’s master antioxidant and has several functions to remove toxins from the body. Using this approach increased the success rate of the program by 25%. A great improvement but still far from ideal.
In 2003, another breakthrough came for Jim Harper. A very high percentage of the people tapering Pristiq would experience a withdrawal symptom call “brain zaps” during the taper. This is a very debilitating sensation, described as a sudden electrical jolt that runs from the base of the neck up into the head. It is often brought on when a person moves their eyes from side to side or up and down. The Pristiq induced brain zaps caused most people to stop their Pristiq taper and just go back higher on the Pristiq for relief and never attempt to taper Pristiq again.
We will not go into the science of how the solution was discovered in this book but provide the solution. Omega 3 fish oil high in EPA is the solution. How much you take and when you take the omega 3 is important as well. Over the years, Jim refined the use of omega 3 to; the best type of fish to use in the oil, when and how much to take. This resulted in using less fish oil as well for the desired results.
In 2004, Jim started a DNA testing company with the idea that our individual DNA held promise in creating an even more successful Pristiq withdrawal program. Initially, Jim tested how the medications affect our DNA, and the role DNA played in the metabolism speed of the drugs. Once again, great results came from this research and you will now find in the description of virtually all medications, which DNA metabolism route the drug takes to breakdown and most importantly, which people should or should not use certain medications if their DNA is a certain way.
As great as this information was, it still did not help with tapering Pristiq. It only showed why some people could take Pristiq and do fine on it and taper it without any problem.
Next, Jim began looking at nutritional supplements, DNA, and tapering. Near the end of 2004, Jim formulated his first supplements that were designed specifically to assist in psychotropic medication withdrawal. Jim sent several bottles to a physician he had been in contact with for several years so he could use them with a group of patients. These patients had been on their psychotropic medication for several years and had not been able to taper of the drug.
All the patients tapered off their medication and did exceptionally well during the taper. All were able to remain off their medication and no original symptoms returned.
Jumping ahead to 2010, Jim discovered a gene that is disrupted when taking any psychotropic medication. The next major breakthrough in tapering followed. Reduce the activation of this gene and withdrawal symptoms fade away and fade away quickly. Over the 15 years since this discovery, Jim has continued his research and the success rate of tapering with his method is still the best in the health community.
This was a short story for you, so you know why Jim recommends specific supplements during the taper. If you choose not to use them, Jim still provides in this book how to taper each type of medication and what to watch out for along your journey.
Life is defined as: the quality that distinguishes a vital and functional being from a dead body or inanimate matter (Webster’s Dictionary). Per the definition of life, you are vital. We need you and humankind needs you. The positive changes you can bring to others are beyond imagination. Life can be grand, life can be fulfilling; you, changing your life and having “reach” return will absolutely affect others in your environment.
Reach can return with your children, spouse, work or activities you have been putting off for years that you have always wanted to do, or to do once again.
Remember and hold the following close to your heart as you travel this journey:
- You Can Change.
- You Can Change How You Feel.
- You Can Be a Positive Influence for Others.
- You Can Make It
My best to all of you on this journey,
James Harper
Chapter 1
Tapering Pristiq
Tapering the Pristiq, with or without using supplements, is the same. There is an exception to this, and it is imperative to mention.
With you taking Pristiq, you will likely need to take omega 3 fish oil. No way around this. When withdrawing from Pristiq you will likely develop what is known as “brain Zaps.” These are electrical type jolts that tend to start in the upper neck area and run up into the skull. Moving your eyes to the side or up and down can make the brain zaps start or become worse. Get an omega 3 fish oil that is higher in EPA than DHA or go to www.ngscart.com and look for the Omega 3 Supreme.
You should take 1 softgel in the morning and 1 softgel at noon. You can adjust the omega 3 around to match what works best for you. See the chapter Using Supplements for more detail on using omega 3 to stop the brain zaps.
Pristiq Withdrawal Timeline
Pristiq should be tapered by no more than 10% every 14 days. If more time is needed to feel stable on a new dosage of Pristiq, give yourself additional time. Slow and steady is going to win this race.
I will say this repeatedly in this book, Slow and steady wins this race.
You are likely taking the 50mg dosage of Pristiq and wondering how to taper at 10% if the Pristiq does not come in those increments. You will need to use a compounding pharmacy to accomplish this.
The pharmacy can make you a 45mg dosage, 40mg dosage, 35mg dosage and so on.
Have the pharmacy make you a 30-day supply of each new dosage. You should do that in case you are having withdrawal side effects on a new lower dosage and need to go back up to the old dosage again to feel stable.
When tapering Pristiq, only reduce to the next dosage when you are very stable on the reduced amount and at least 14 days have passed. It will be very rare for me to ever say, “I don’t care what your physician says to you” but this is one occasion where “I just do not care.” DO NOT REDUCE PRISTIQ FASTER THAN THIS AND DO NOT REDUCE BY MORE THAN 10%.
The drug manufacturer will state Pristiq must be reduced slowly and gradually. This statement does not say, it is assumed the Pristiq should be reduced slowly and gradually, it says the drug should be.
There is a slight chance the 10% reduction will be too fast for you, and you will need to taper Pristiq by 5%. There is a reason for that, and it is discussed in the chapter General Information.
Never skip days taking the Pristiq when tapering.
Continue taking the Pristiq at the same time each day.
Do not quit smoking or start a diet when you are tapering Pristiq.
Avoid major life changes while tapering Pristiq.
Chapter 2
Pristiq Withdrawal Symptoms
This chapter lists many possible side effects experienced from Pristiq, or when trying to withdraw from Pristiq.
You may know from experience that a single withdrawal side effect can be horrifying. And if you, or anyone you know, have ever had a bad withdrawal experience you would probably rather sign up for open-heart surgery without anesthesia than suffer those side effects again. And for this very reason, many people who have contacted The Road Back are gun shy at the very thought of withdrawing from Pristiq. Before The Road Back, you were faced with a quandary: suffer the side effects of Pristiq or gut it out and suffer the side effects of withdrawal.
One thing to keep in mind while doing this program or with any inpatient program you might enroll in, if you have a bad day and feel out of sorts, have a headache, ache or a pain, do not sleep well etc., these feelings or symptoms may not be withdrawal. We all have bad days from time to time and how you feel out of the blue can be quite normal. This can be difficult when you have had insomnia for months and begin to sleep better and then out of the blue you have a difficult night sleeping. If the insomnia lasts for more than 3 nights then something needs to be done, but an occasional restless night or sleepless night is common.
This past year we had a person call us and she described how she has had a headache for the past 4 days and how it came out of nowhere. She was ½ way off her medication and doing very well and she felt this was a withdrawal side effect and wanted to know what to do. After a little communication and looking for changes that might have taken place, we found out her best friend had died unexpectedly the day before the headaches started. This might seem easy to spot as a reason for the headaches, but when you are in the middle of withdrawal and you have suffered extreme side effects in the past, it can be easy to lose track and worry about the slightest changes in how you feel.
As you read through the list of potential Pristiq side effects in this chapter, do keep in mind these emotional and physical conditions existed long before the first Pristiq was manufactured. We are only dealing with drug-induced side effects with this program.
This program helps to eliminate these worries and concerns by reducing the side effects of withdrawal, so that you can come off Pristiq smoothly and easily.
The following list is broken down into categories, covering the various areas of the body, such as the nervous system, lymph system, emotional and mental symptoms and so forth. These categories will make it easier for you to find the part of the body or system that you are interested in or want to know more about.
In this list, you will find many physical ailments and complaints, as well as emotional or mental symptoms that people experience every day because of a specific medical condition. These symptoms and ailments may be the reason that you started using a drug, or conversely, these drugs may be causing the negative symptoms you are experiencing now.
This unknown catches almost everyone, doctor and patient alike, off guard. So, the question that needs to be answered for you to proceed with this program is: Are you dealing with a physical condition that needs to be treated medically or with a by-product symptom of the drug(s) you are taking?
Getting Your Doctor’s Approval
Because of the overload and damage potentially caused by Pristiq, your body in general, and your immune system in particular, are in a weakened condition, and can thus leave you open to infections and disease. On the other hand, you may be taking prescription medications for actual physical conditions, which could be contra-indicated or need to be closely monitored in terms of doing this program. These could include blood thinners and heart medication, as well as clotting agents.
For these reasons, consult your doctor before starting any part of this program to sort out, or discover and correctly determine whether you are a candidate for this program.
After you have ruled out any real medical problem, you will know that if any strange symptoms begin during this program, you are most likely experiencing something caused by Pristiq. Such will be true for both emotional and physical symptoms.
The following list does not include all possible side effects from Pristiq, this book would need thousands of pages if this were undertaken. Using the Freedom of Information Act, I received all side effects associated with Pristiq during clinical trials. That list alone is long enough to make this book double the size if they are included. The side effects in this chapter are the most common.
Highlight or make a mark by existing symptoms you currently have in the list below.
Dry Mouth – Less moisture in the mouth than is usual.
Increased Sweating – A large quantity of perspiration that is medically caused.
Allergy – Extreme sensitivity of body tissues triggered by substances in the air, drugs, or foods causing a variety of reactions such as sneezing, itching, asthma, hay fever, skin rashes, nausea and/or vomiting.
Asthenia – A physically weak condition.
Chest Pains – Severe discomfort in the chest caused by not enough oxygen going to the heart because of blood vessel narrowing or spasms.
Chills – Appearing pale while cold and shivering. Sometimes accompanied by fever.
Edema of Extremities – Abnormal swelling of body tissue caused by the collection of fluid.
Fall – Suddenly losing a normal standing upright position.
Fatigue – Loss of normal strength thus not able to do usual physical and mental activities.
Fever – Abnormally high body temperature, normal being 98.6 degrees Fahrenheit or 37 degrees Centigrade. Fever is a symptom of disease or disorder in the body. The body is affected by feeling hot, chilled, sweaty, weak and exhausted. If the fever goes too high or lasts too long, death can result.
Hot Flashes – Brief, abnormal enlargement of blood vessels that causes a sudden heat sensation over the entire body. Sometimes experienced by menopausal women.
Influenza (Flu)-like Symptoms – Demonstrating irritation of the respiratory tract (organs of breathing) such as a cold, sudden fever, aches and pains, as well as feeling weak and seeking bed rest, which is similar to having the flu.
Leg Pain – A hurtful sensation in the legs caused by excessive stimulation of the nerve endings in the legs, resulting in extreme discomfort.
Malaise – The somewhat unclear feeling of discomfort when a person starts to feel sick.
Pain in Limb – Sudden, sharp and uncontrolled leg or arm discomfort.
Syncope – A short period of light-headedness or unconsciousness (black- out) also known as fainting, caused by lack of oxygen to the brain because of an interruption in blood flow to the brain.
Tightness of Chest – Mild or sharp discomfort, tightness or pressure in the chest area (anywhere between the throat and belly). The causes can be mild or seriously life-threatening because they include the heart, lungs and surrounding muscles.
CARDIOVASCULAR
(INVOLVING THE HEART AND THE BLOOD VESSELS)
Palpitation – Unusual and abnormal heartbeat that is sometimes irregular, but rapid, and forceful thumping or fluttering. It can be brought on by shock, excitement, exertion or medical stimulants. A person is normally unaware of his/her heartbeat.
Hypertension – High blood pressure, a symptom of disease in the blood vessels leading away from the heart. Hypertension is known as the “silent killer.” The symptoms are usually not obvious; however, it can lead to damage to the heart, brain, kidneys and eyes, and can even lead to stroke and kidney failure.
Bradycardia – The heart rate is slowed from around 72 beats per minute, which is normal, to below 60 beats per minute in an adult.
Tachycardia – The heart rate speeds up to above 100 beats per minute in an adult. Normal adult heart rate is 72 beats per minute.
ECG Abnormal – A test called an electrocardiogram (ECG) records the activity of the heart by measuring heartbeats as well as the position and size of the heart’s four chambers. An ECG also measures whether there is damage to the heart and the effects of drugs or mechanical devices like a heart pacemaker. When the test is abnormal this means one or more of the following are present: heart disease, defects, beating too fast or too slow, disease of the blood vessels leading from the heart or the heart valves, and/or a past or impending heart attack.
Flushing – Skin all over the body turns red.
Varicose Veins – Unusually swollen veins near the surface of the skin that sometimes appear twisted and knotted but always enlarged. They are called hemorrhoids when appearing around the rectum. The cause is attributed to hereditary weakness in the veins aggravated by obesity, pregnancy, pressure from standing, aging, etc. Severe cases may develop swelling in the legs, ankles and feet, eczema and/or ulcers in the affected areas.
GASTROINTESTINAL
Abdominal Cramp/Pain – Sudden, severe, uncontrollable and painful shortening and thickening of the muscles in the belly. The belly includes the stomach, as well as the intestines, liver, kidneys, pancreas, spleen, gall bladder and urinary bladder.
Belching – Noisy release of gas from the stomach through the mouth.
Bloating – Swelling of the belly caused by excessive intestinal gas.
Constipation – Difficulty in having a bowel movement where the material in the bowels is hard due to a lack of exercise, fluid intake, or roughage in the diet or due to certain drugs.
Diarrhea – Unusually frequent and excessive runny bowel movements that may result in severe dehydration and shock.
Dyspepsia/Indigestion – The discomfort one may experience after eating. It can be heartburn, gas, nausea, a bellyache or bloating.
Flatulence – More gas than normal in the digestive organs.
Gagging – Involuntary choking and/or involuntary vomiting.
Gastritis – A severe irritation of the mucus lining of the stomach, either short in duration or lasting for a long period of time.
Gastroenteritis – A condition in which the membranes of the stomach and intestines are irritated.
Gastrointestinal Hemorrhage – Excessive internal bleeding in the stomach and intestines.
Gastro Esophageal Reflux – A continuous state where stomach juices flow back into the throat causing acid indigestion and heartburn and possibly injury to the throat.
Heartburn – A burning pain around the breastbone caused by stomach juices flowing back up into the throat.
Hemorrhoids – Small rounded purplish swollen veins that bleed, itch or are painful and appear around the anus.
Increased Stool Frequency – see “Diarrhea.”
Indigestion – Inability to properly consume and absorb food in the digestive tract, causing constipation, nausea, stomachache, gas, swollen belly, pain and general discomfort or sickness.
Nausea – Stomach irritation with a queasy sensation like motion sickness and a feeling that one is going to vomit.
Polyposis Gastric – Tumors that grow on stems in the lining of the stomach, which usually become cancerous.
Swallowing Difficulty – A feeling that food is stuck in the throat or upper chest area and won’t go down, making it
difficult to swallow.
Toothache – Pain in a tooth above and below the gum line.
Vomiting – Involuntarily throwing up the contents of the stomach, usually accompanied by a nauseated, sick feeling just prior to doing so.
HEMIC & LYMPHATIC
(INVOLVING THE BLOOD AND THE CLEAR FLUIDS IN THE TISSUES THAT CONTAIN WHITE BLOOD CELLS)
Anemia – A condition in which the blood is no longer carrying enough oxygen, so the person looks pale and easily gets dizzy, weak and tired. More severely, a person can end up with an abnormal heart, as well as breathing and digestive difficulties.
Bruise – Damage to the skin resulting in a purple-green- yellow skin coloration that is caused by breaking of the blood vessels in the area without breaking the surface of the skin.
Nosebleed – Blood loss from the nose.
Hematoma – Broken blood vessels that cause a swelling in an area on the body.
Lymphadenopathy Cervical – The lymph nodes in the neck, part of the body’s immune system, become swollen and enlarged by reacting to the presence of a drug. The swelling is the result of the white blood cells multiplying to fight the invasion of the drug.
METABOLIC & NUTRITIONAL
(ENERGY AND HEALTH)
Arthralgia – Sudden sharp nerve pain in one or more joints.
Arthropathy – Joint disease or abnormal joints.
Arthritis – Painfully inflamed and swollen joints. The reddened and swollen condition is brought on by a serious injury or shock to the body either from physical or emotional causes.
Back Discomfort – Severe physical distress in the area from the neck to the pelvis along the backbone.
Bilirubin Increased – Bilirubin is a waste product of the breakdown of old blood cells. Bilirubin is sent to the liver to be made water-soluble so it can be eliminated from the body through emptying the bladder. A drug can interfere with or damage this normal liver function, creating liver disease.
Decreased Weight – Uncontrolled and measured loss of heaviness or weight.
Gout – A severe arthritis condition that is caused by the dumping of a waste product called uric acid into the tissues and joints. It can worsen and cause the body to develop a deformity after going through stages of pain, inflammation, severe tenderness and stiffness.
Hepatic Enzymes Increased – An increase in the amount of paired liver proteins that regulate liver processes causing a condition in which the liver functions abnormally.
Hypercholesterolemia – Too much cholesterol in the blood cells.
Hyperglycemia – An unhealthy amount of sugar in the blood.
Increased Weight – A concentration and storage of fat in the body accumulating over a period of time caused by unhealthy eating patterns, a lack of physical activity, or an inability to process food correctly, which can predispose the body to many disorders and diseases.
Jaw Pain – Pain due to irritation and swelling of the nerves associated with the mouth area where it opens and closes just in front of the ear. Some of the symptoms are pain when chewing, headaches, loss of balance, stuffy ears or ringing in the ears and teeth grinding.
Jaw Stiffness – The result of squeezing and grinding the teeth while asleep that can cause teeth to deteriorate, as well as the muscles and joints of the jaw.
Joint Stiffness – A loss of free motion and easy flexibility.
Muscle Cramp – When muscles contract uncontrollably without warning and do not relax. The muscles of any body organs can cramp.
Muscle Stiffness – The tightening of muscles makes it difficult to bend.
Muscle Weakness – Loss of physical strength.
Myalgia – A general widespread pain and tenderness of the muscles.
Thirst – A strong, unnatural craving for moisture/water in the mouth and throat.
NERVOUS SYSTEM (SENSORY CHANNELS)
Carpal Tunnel Syndrome – A pinched nerve in the wrist that causes pain, tingling, and numbing.
Coordination Abnormal – A lack of normal, harmonious interaction of the parts of the body when it is in motion.
Dizziness – Losing one’s balance while feeling unsteady and lightheaded. May lead to fainting.
Disequilibrium – Lack of mental and emotional balance.
Faintness – A temporary condition in which one is likely to become unconscious and fall.
Headache – A sharp or dull persistent pain in the head.
Hyperreflexia – A not normal (abnormal) and involuntary increased response in the tissues connecting the bones to the muscles.
Light-Headed Feeling – An uncontrolled and usually brief loss of consciousness usually caused by a lack of oxygen to the brain.
Migraine – Recurring severe head pain sometimes accompanied by nausea, vomiting, dizziness, flashes or spots before the eyes and ringing in the ears.
Muscle Contractions Involuntary – A spontaneous and uncontrollable tightening reaction of the muscles caused by electrical impulses from the nervous system.
Muscular Tone Increased – Uncontrolled and exaggerated muscle tension. Muscles are normally partially tensed which is what gives muscle tone.
Paresthesia – Burning, prickly, itchy, or tingling skin with no obvious or understood physical cause.
Restless Legs – A need to move the legs without any apparent reason. Sometimes there is pain, twitching, jerking, cramping, burning or a creepy-crawly sensation associated with the movements. It worsens when a person is inactive and can interrupt sleep so one feels the need to move to gain some relief.
Shaking – Uncontrolled quivering and trembling as if one is cold and chilled.
Sluggishness – Lack of alertness and energy, as well as being slow to respond or perform in life.
Tics – A contraction of a muscle causing a repeated movement not under the control of the person, usually on the face or limbs.
Tremor – A nervous and involuntary vibrating or quivering of the body.
Twitching – Sharp, jerky and spastic motion, sometimes with sharp sudden pain.
Vertigo – A sensation of dizziness with disorientation and confusion.
MENTAL AND EMOTIONAL
Aggravated Nervousness – A progressively worsening, irritated, and troubled state of mind.
Agitation – A suddenly violent and forceful emotionally disturbed state of mind.
Amnesia – Long or short term, partial or full memory loss created by emotional or physical shock, severe illness, or a blow to the head where the person was caused pain and became unconscious.
Anxiety Attack – Sudden and intense feelings of fear, terror, and dread, physically creating shortness of breath, sweating, trembling and heart palpitations.
Apathy – Complete lack of concern or interest for things that ordinarily would be regarded as important or would normally cause concern.
Appetite Decreased – Lack of appetite despite the ordinary caloric demands of living, with a resulting unintentional loss of weight.
Appetite Increased – An unusual hunger causing one to overeat.
Auditory Hallucination – Hearing things without the voices or noises being present.
Bruxism – Grinding and clenching teeth while sleeping.
Carbohydrate Craving – A drive or craving to eat foods rich in sugar and starches (sweets, snacks and junk foods) that intensifies as the diet becomes more and more unbalanced due to the unbalancing of the proper nutritional requirements of the body.
Concentration Impaired – Unable to easily focus attention for long periods of time.
Confusion – Inability to think clearly or understand, preventing logical decision making.
Crying (Abnormal) – Unusual fits of weeping for short or long periods of time for no apparent reason.
Depersonalization – A condition in which one has lost a normal sense of personal identity.
Depression – A hopeless feeling of failure, loss and sadness that can deteriorate into thoughts of death. A very common reaction to or side effect of psychoactive drugs.
Disorientation – A loss of sense of direction, place, time or surroundings, as well as mental confusion regarding one’s personal identity.
Dreaming (Abnormal) – Dreaming that leaves a very clear, detailed picture and impression when awake that can last for a long period of time and sometimes be unpleasant.
Emotional Lability – Suddenly breaking out in laughter or crying or doing both without being able to control the outburst of emotion. These episodes are unstable as they are caused by experiences or events that normally would not have this effect on an individual.
Excitability – Uncontrollably responding to stimuli (one’s environment).
Feeling Unreal – The awareness that one has an undesirable emotion like fear but can’t seem to shake off the irrational feeling. For example, feeling like one is going crazy, but rationally knowing that it is not true. Resembles experiencing a bad dream and not being able to wake up.
Forgetfulness – Unable to remember what one ordinarily would remember.
Insomnia – Sleeplessness caused by physical stress, mental stress or stimulants, such as coffee or medications, awake when one would ordinarily be able to fall and remain asleep.
Irritability – An abnormal reaction of being annoyed or disturbed in response to a stimulus in the environment.
Jitteriness – Nervous fidgeting without apparent cause.
Lethargy – Mental and physical sluggishness and apathy (a feeling of hopelessness that “nothing can be done”) which can deteriorate into an unconscious state resembling deep sleep. A numb state of mind.
Libido Decreased – An abnormal loss of sexual energy or desire.
Panic Reaction – A sudden, overpowering, chaotic and confused mental state of terror resulting in doubt-ridden, often accompanied by hyperventilation and extreme anxiety.
Restlessness Aggravated – A constantly worsening troubled state of mind characterized by increased nervousness, inability to relax and quick temper.
Somnolence – Feeling sleepy all the time or having a condition of semi- consciousness.
Suicide Attempt – An unsuccessful deliberate attack on one’s own life with the intention of ending it.
Suicidal Tendency – Most likely will attempt to kill oneself.
Tremulousness Nervous – Very jumpy, shaky, and uneasy, while feeling fearful and timid. The condition is characterized by dread of the future, involuntary quivering, trembling, and feeling distressed and suddenly upset.
Yawning – Involuntary opening of the mouth with deep inhalation of air.
REPRODUCTIVE FEMALE
Breast Neoplasm – A tumor or cancer, of either of the two milk-secreting organs on the chest of a woman.
Menorrhagia – Abnormally heavy menstrual period or a menstrual flow that has continued for an unusually long period of time.
Menstrual Cramps – Painful, involuntary uterus contractions that women experience around the time of their menstrual period, sometimes causing pain in the lower back and thighs.
Menstrual Disorder – A disturbance or derangement in the normal function of a woman’s menstrual period.
Pelvic Inflammation – The reaction of the body to infectious, allergic or chemical irritation, which, in turn, causes tissue irritation, injury, or bacterial infection characterized by pain, redness, swelling, and sometimes loss of function. The reaction usually begins in the uterus and spreads to the fallopian tubes, ovaries and other areas in the hipbone region of the body.
Premenstrual Syndrome – Various physical and mental symptoms commonly experienced by women of childbearing age usually 2 to 7 days before the start of their monthly period. There are over 150 symptoms including eating binges, behavioral changes, moodiness, irritability, fatigue, fluid retention, breast tenderness, headaches, bloating, anxiety and depression. The symptoms cease shortly after the period begins and disappear with menopause.
Spotting Between Menses – Abnormal bleeding between periods. Unusual spotting between menstrual cycles.
RESPIRATORY SYSTEM
Asthma – A disease of the breathing system initiated by an allergic reaction or a chemical, with repeated attacks of coughing, sticky mucus, wheezing, shortness of breath and a tight feeling in the chest. The disease can reach a state where it stops a person from exhaling, leading to unconsciousness and death.
Breath Shortness – Unnatural breathing, using a lot of effort resulting in not having enough air taken in by the body.
Bronchitis – Inflammation of the two main breathing tubes leading from the windpipe to the lungs. The disease is marked by coughing, a low-grade fever, chest pain and hoarseness. It can also be caused by an allergic reaction.
Coughing – A cough is the response to irritation, such as mucus, that causes the muscles to control the breathing process to expel air from the lungs suddenly and noisily to keep the air passages free from the irritating material.
Laryngitis – Inflammation of the voice box characterized by hoarseness, sore throat, and coughing. It can be caused by straining the voice or exposure to infectious, allergic or chemical irritation.
Nasal Congestion – The presence of an abnormal amount of fluid.
Pneumonia Tracheitis – Bacterial infection of the air passageways and lungs that causes redness, swelling and pain in the windpipe. Other symptoms are high fever, chills, pain in the chest, difficulty breathing and coughing with mucus discharge.
Rhinitis – Chemical irritation causing pain, redness and swelling in the mucus membranes of the nose.
Sinus Congestion – The mucus-lined areas of the bones in the face that are thought to help warm and moisten air to the nose. These areas become clogged with excess fluid or become infected.
Sinus Headache – An abnormal amount of fluid in the hollows of the facial bone structure, especially around the nose. This excess fluid creates pressure, causing pain in the head.
Sinusitis – The body reacts to chemical irritation causing redness, swelling and pain in the area of the hollows in the facial bones, especially around the nose.
SKELETAL
Neck/Shoulder Pain – Hurtful sensations of the nerve endings caused by damage to the tissues in the neck and shoulder, signaling danger of disease.
SKIN AND APPENDAGES (SKIN, LEGS AND ARMS)
Acne – Eruptions of the oil glands of the skin, especially on the face, marked by pimples, blackheads, whiteheads, bumps and more severely, by cysts and scarring.
Alopecia – The loss of hair, baldness.
Angioedema – Intense itching and swelling welts on the skin called hives caused by an allergic reaction to internal or external agents. The reaction is common to a food or a drug. Chronic cases can last for a long period of time.
Dermatitis – Generally irritated skin that can be caused by any of a number of irritating conditions, such as parasites, fungus, bacteria, or foreign substances causing an allergic reaction. It is a general inflammation of the skin.
Dry Lips – The lack of normal moisture in the fleshy folds that surround the mouth.
Dry Skin – The lack of normal moisture/oils in the surface layer of the body. The skin is the body’s largest organ.
Epidermal Necrolysis – An abnormal condition in which a large portion of the skin becomes intensely red and peels off like a second-degree burn. Symptoms often include blistering.
Eczema – A severe or continuing skin disease marked by redness, crusting and scaling, with watery blisters and itching. It is often difficult to treat and will sometimes go away only to reappear again.
Folliculitis – Inflammation of a follicle (small body sac), especially hair follicle.
Furunculosis – Skin boils that show up repeatedly.
Lipoma – A tumor of mostly fat cells that is not health endangering.
Pruritus – Extreme itching of often-undamaged skin.
Rash – A skin eruption or discoloration that may or may not be itching, tingling, burning or painful. It may be caused by an allergy, skin irritation or a skin disease.
Skin Nodule – A bulge, knob, swelling or growth in the skin that is a mass of tissue or cells.
RELATED TO THE SENSES
Conjunctivitis – Infection of the membrane that covers the eyeball and lines the eyelid, caused by a virus, allergic reaction or an irritating chemical. It is characterized by redness, a discharge of fluid and itching.
Dry Eyes – Not enough moisture in the eyes.
Earache – Pain in the ear.
Eye Infection – The invasion of the eye tissue by a bacteria, virus, fungus, etc., causing damage to the tissue, with toxicity. Infection spreading in the body progresses into disease.
Eye Irritation – An inflammation of the eye.
Metallic Taste – A range of taste impairment from distorted taste to a complete loss of taste.
Pupils Dilated – Abnormal expansion of the black circular opening in the center of the eye.
Taste Alteration – Abnormal flavor detection in food.
Tinnitus – A buzzing, ringing or whistling sound in one or both ears occurring from the use of certain drugs.
Vision Abnormal – Normal images are seen differently by the viewer than by others.
Vision Blurred – Eyesight is dim or indistinct and hazy in outline or appearance.
Visual Disturbance – Eyesight is interfered with or interrupted. Examples of disturbances are light sensitivity and the inability to easily distinguish colors.
URINARY SYSTEM
Blood in Urine – Blood is present when one empties the liquid waste product of the kidneys through the bladder by urinating in the toilet, turning the water pink to bright red. Or spots of blood are observable in the water after urinating.
Dysuria – Difficult or painful urination.
Kidney Stone – Small hard masses of salt deposits that the kidney forms.
Urinary Frequency – Having to urinate more often than usual or between unusually short time periods.
Urinary Tract Infection – An invasion of bacteria, viruses, fungi, etc., of the system in the body. This starts with the kidneys, which eliminate urine from the body. If the invasion goes unchecked, it can injure tissue and progress into disease.
Urinary Urgency – A sudden compelling urge to urinate, accompanied by discomfort in the bladder.
UROGENITAL (URINARY TR ACT)
Anorgasmia – Failure to experience an orgasm.
Ejaculation Disorder – Dysfunction during orgasm.
Menstrual Disorder – Dysfunction of the discharge during the monthly menstrual cycle.
VIOLENT OR PHYSICALLY DANGEROUS SIDE EFFECTS
Acute Renal Failure – The kidneys stop excreting waste products properly, leading to rapid poisoning (toxicity) in the body.
Anaphylaxis – A violent, sudden, and severe drop in blood pressure caused by a re-exposure to a foreign protein or a second dosage of a drug that may be fatal unless emergency treatment is given right away.
Grand Mal Seizures (or Convulsions) – A recurring sudden, violent and involuntary attack of muscle spasms with a loss of consciousness.
Neuroleptic Malignant Syndrome – A life threatening, rare reaction to an anti-psychotic drug marked by fever, muscular rigidity, changed mental status and dysfunction of the autonomic nervous system.
Pancreatitis – Chemical irritation with redness, swelling and pain in the pancreas where digestive enzymes and hormones are secreted.
QT Prolongation – A very fast heart rhythm disturbance that is too fast for the heart to beat effectively so the blood to the brain falls, causing a sudden loss of consciousness. May cause sudden cardiac arrest.
Rhabdomyolysis – The breakdown and release of muscle fibers into the circulatory system.
Serotonin Syndrome – A disorder brought on by excessive levels of serotonin. Symptoms include euphoria, drowsiness, sustained and rapid eye movement, agitation, reflexes overreacting, rapid muscle contractions, abnormal movements of the foot, clumsiness, feeling drunk and dizzy without any intake of alcohol, jaw muscles contracting and relaxing excessively, muscle twitching, high body temperature, ridged body, rotating mental status – including confusion and excessive happiness – diarrhea and loss of consciousness.
Thrombocytopenia – An abnormal decrease in the number of blood platelets in the circulatory system. A decrease in platelets causes a decrease in the ability of the blood to clot when necessary.
Torsades de Pointes – Unusually rapid heart rhythm starting in the lower heart chambers. If the short bursts of rapid heart rhythm continue for a prolonged period, it can degenerate into a more rapid rhythm and can be fatal.
Chapter 3
Taper Using Supplements
When you taper using the suggested supplements, taper the Pristiq the same as suggested in chapter 1.
If you are currently having strong withdrawal before starting this taper method, you must go back up to the last dosage of Pristiq where you felt stable. There is no way around this.
Do this while you are waiting for the supplements to arrive. The supplements are found at www.ngscart.com/products/package-c
Neuro Genetic Solutions has created packages for you, so you do not need to order the supplements individually.
The supplements you will need are Maca Supreme, JNK Formula Complete, Omega 3 Supreme, Neuro Day and Neuro Night. It’s advisable that you order an extra 2 bottles of Maca Supreme.
It’s advisable you order an extra 2 bottles of Maca Supreme and 2 extra bottles of Omega 3 Supreme.
How to Take Supplements
Maca Supreme – It is important you start the Maca Supreme exactly as described in this book.
Take 1 capsule at 8am each day.
4 days later take an additional capsule at 12pm noon.
4 days later take 2 capsules at 8am.
4 days later take 2 capsules at 12pm noon.
Your schedule may not allow you to take the Maca Supreme exactly at 8am or at 12pm noon. If that is the case, take as close to these times as possible.
JNK Formula Complete – After you have taken the 4 Maca Supreme capsules as described above for 4 consecutive days, you are ready to start the JNK Formula and the rest of the supplements.
Take 1 JNK Formula capsule with the 8am Maca Supremes. Take a second JNK Formula with the 12pm noon Maca Supremes.
Neuro Day – Take 1 capsule of the Neuro Day at 10am. Take a second Neuro Day at 2pm.
Neuro Night – Take 2 capsules of Neuro Night about 30 minutes before bedtime.
Omega 3 Supreme – To begin, take 1 softgel each time you take a JNK Formula capsule. You may need to adjust the time you take the Omega 3 Supreme once you begin tapering again.
If you tend to have brain zaps start first thing in the morning, take 1 Omega 3 Supreme when you wake up in the morning. The idea is to look for a trend of the start times of a brain zap and take Omega 3 Supreme at least 30 minutes before they tend to start. This might be in the morning or the afternoon.
With the first reducing of an antidepressant, you may need to take 2 of the Omega 3 Supreme at the same time to get rid of the brain zaps. If you had to go back up on the antidepressant dosage to get rid of the brain zaps, start taking 2 softgels in the morning. You need to eliminate the brain zaps before you restart a taper again.
Restarting the Taper
If you have been tapering the medication before starting this withdrawal method, if you have withdrawal side effects that are in the moderate range to extreme withdrawal, go back up to the last dosage of the medication you were taking that did not have these withdrawal side effects.
While you do this, get the supplements.
When the supplements arrive begin taking them as described.
Take all the supplements for 2 full weeks before resuming the taper again. Then follow the chapter on how to reduce the medications.
You continue taking the supplements throughout the taper. You should continue taking the supplements for 30 days after your last dose of the medication.
It is fine to continue taking the supplements for life if you wish. Many people take one or more of the supplements daily to help with anxiety or sleep.
If Starting the Taper for the First Time
Keep taking the medication at the dosage you have been, and start the supplements exactly as described. Once you have taken the supplements for 14 days you should be ready to start reducing the medication.
Before you start to taper have a look at the graphs you have been keeping and look at the side effects you may still have. Have they greatly reduced? If they have not reduced to a minimal point yet, continue the supplements for one more week and then have another look at the graphs.
If they have not greatly reduced at this point it is time for you to send an email to Jim Harper at Jim@theroadback.org and he will help sort out what is happening.
Only reduce the medication every 14 days and only reduce again if you are feeling very well.
Daily Supplement and Medication Schedule
Keep taking the medication at the same time each day throughout the taper.
Do not take a supplement within 1 hour of when you take the medication.
Maca Supreme – 2 capsules at 8am and 2 at 12 noon.
JNK Formula Complete – Take 1 JNK with the 8am Maca Supreme and 1 capsule with the 12 noon Maca Supreme.
Neuro Day – Take 1 capsule at 10am and 1 capsule at 2pm.
Neuro Night – Take 2 capsules 30 minutes before bedtime.
Omega 3 Supreme – If taking an antidepressant, Take 1 softgel with the morning Maca Supreme and the 12 noon Maca Supreme.
Chapter 4
Journal
The journal is used to note major changes during the taper. Each time you reduce the medication make a note of the date and what dosage you dropped from and to what new lower dosage.
Make note of any diet changes.
Make note of any physical activity.
You do not need to go overboard while keeping a journal, but it is imperative you keep track of changes you have made.
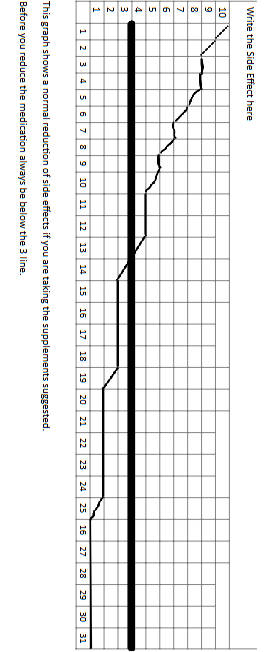
Chapter 5
Graph
Ideally you will create several graphs. One graph for each major side effect you currently have. Go back to Chapter 2, Pristiq Side Effects and make a graph for the major withdrawal side effects you have.
The most common will be anxiety, fatigue, insomnia, body aches and pains.
Get some graph paper and create each graph as provided in the following example.
Write the side effect on the top of the graph, number it from 1-31 and on the left side of the graph you will number from 1-10.
The number 10 should be the most extreme feeling and under number 1 there will be no side effect.
Notice the bolder line at number 3. Any side effect you have should be at a 3 or lower before tapering the Pristiq further.
Chapter 6
General Information
This chapter provides general tips and suggestions.
If you have experienced sexual dysfunction while taking Pristiq, try taking the Maca Supreme as described in the supplement chapter. Give it 30 days and see what changes!
If you are reading this book and are in withdrawal, you need to go back up to the last dosage of the Pristiq you felt stable at and remain there while you decide your next course of action.
If you are already off the Pristiq and still suffering from withdrawal, most likely that withdrawal effect will not stop on its own. Get with your prescribing physician and discuss going back on the medication again. Normally, if you had a dose of the medication, you felt stable during the taper, going back to that dosage will bring relief. I strongly urge you to get the supplements as quickly as possible.
Tapering is one of those things, where doing the same thing over and over and expecting a different result comes to mind. If you have been tapering and suffering withdrawal, unless you change what you have been doing, do not expect a different result.
Slow and steady will win this race.
Pristiq Metabolism
Pristiq uses a liver enzyme to metabolize. Pristiq is extensively metabolized by the liver enzyme CYP3A4. Before going further, I want to detail a bit what this enzyme is, and potential negative and positive.
Your DNA determines how much enzyme fluid you will have in the CYP3A4.
This determines how fast or slow the Pristiq can move through the liver.
Some antidepressants use other liver enzymes to metabolize, and it is a positive that Pristiq only uses the CYP3A4.
These CYP enzymes do not all contain the same amount of enzyme fluid. The CYP3A4 contains the most fluid. Get the idea of a pie and cutting the pie into slices. With the CYP enzymes, not all pieces of the pie are the same size. Some are very small and some larger. The smaller the pie, it only takes a slight change and whatever is trying to move through this slice of pie, enzyme fluid, could have a difficult time going through.
The one main thing that can disrupt the metabolism of Pristiq is high fat meals. This can alter the metabolism rate of Pristiq by as much as 16%. So, if you eat a high fat meal the current dosage of Pristiq you are taking will feel like it is 16% more. You may feel overdosed.
This is why it is not good to diet or change your diet at all when you are tapering Pristiq.
If you are taking any other medication that uses the CYP3A4 to metabolize and you start tapering the Pristiq, the other medication metabolism rate will also change. Since the two drugs are competing for the same pathway, there is no way the other drug will not also be changing how fast it breaks down in the liver.
These are some reasons you may need to taper Pristiq at 5 percent and not 10 percent.
If you are going to use the supplements suggested, special care has been made to ensure the ingredients of each supplement will not affect the metabolism of Pristiq or other medication that use the CYP enzymes for metabolism.
Again, slow and steady wins this race.
The good news. Pristiq is normally not a difficult medication to taper off when tapering slowly and gradually.
During Pristiq withdrawal, any and all head symptoms can be handled by taking Omega 3 Supreme.
Stomach upset can normally be handled by drinking a few cups of ginger tea.
If you did not compound the Pristiq to get a 10 percent reduction and are just reducing to the next available lower dosage and side effects are too much for you, the thing to try are the supplements suggested.
Make sure to go back up to the last dosage you were feeling stable at before the withdrawal side effects have a chance to really take hold though. Once they do, it is more difficult to taper.
There is a chance your physician will want to prescribe you a different antidepressant if Pristiq withdrawal is too much. Normally they select Prozac.
I would avoid this completely.
You will be taken off Pristiq abruptly and put on Prozac. If you were already having withdrawal from Pristiq while tapering the drug, what do you think will happen if you stop Pristiq cold turkey?
The best you can hope for is the Prozac masks the Pristiq withdrawal, and the Prozac does not cause you problems. You are then in a definite fix. Even this program has a difficult time undoing that.
Use Jim Harper for assistance if you need some. Email Jim@theroadback.org
Jim also likes to hear about your successes.
About the Author
In 1999, our founder, Jim Harper, began investigating antidepressants and the cause of their adverse reactions. In 1999, the Internet had two distinct types of websites about psychotropic drugs, those that only stated how bad the drugs were and those sites that only promoted their usage. While others were arguing about the medications, Jim Harper began looking for solutions for withdrawal. Eventually, most every person will reach a point where the physician and patient will want to discontinue the drug and withdrawal assistance needed to be created. In 1999, Jim Harper’s program was the first psychotropic drug withdrawal program.
After 26 years and over 19 million using the program, The Road Back Program is the most widely used drug withdrawal program in the world. The Road Back is a past member of the California Association of Alcoholism & Drug Abuse Counselors (CAADAC).
Jim Harper used the information gathered from his DNA Testing company, Advanced DNA Testing, to advance the program continually over the past decades. After conducting hundreds of DNA test to determine how individuals metabolize psychotropic drugs, evaluating the potential side effects we can all experience due to medications, a basic program was developed. Once DNA testing was completed with hundreds of people regarding their ability to metabolize nutrients and drugs, the program made its next advancement.
Currently, specific supplements are used with The Road Back Program to target genes the drugs activate. The simple process of switching this drug induced activations off again brings relief to the patient during withdrawal.
From all of us at The Road Back – We wish you the best on your journey.
Jim Harper has never used psychotropic medications and is not a recovered addict. Jim has owned a DNA testing company, has been approved by the Los Angeles, CA. courts to be an expert DNA witness and has authored 16 bestselling books about drug withdrawal. One of Jim’s books was used as a textbook at a major university in America in their pharmacology doctoral program.
Jim does understand what you are going through. He has personally answered hundreds of thousands of e-mails over the past 26 years and will answer yours as well and guide you through the withdrawal process.
You will find written in the drug manufacturers description of psychotropic drugs a statement of how to reduce the medication. In 1999, Jim published how to reduce addictive medications and Jim’s approach from 1999 is now recommended by the drug companies. “Reduce the drug slowly and gradually. If side effects become too unbearable, go back up to the last dosage you were doing fine with, get stable once again, and then resume the reduction at a more gradual pace.”
Pristiq withdrawal – How to taper Pristiq is fully described in How to Taper Psychoactive Medication. The full step by step process in detail. Free assistance is also available if needed.
Pristiq Withdrawal Symptoms – What are the symptoms of Pristiq withdrawal described as well as how to eliminate Pristiq withdrawal symptoms.
Pristiq withdrawal – What is the Pristiq withdrawal timeline? You will find that in How to Taper Psychoactive Medication. There is a timeline you should follow for a successful Pristiq withdrawal.
Pristiq withdrawal – Headache is all too common during Pristiq withdrawal. Want the solution? It is found in How to Taper Psychoactive Medication.
Pristiq withdrawal – Some have anxiety during Pristiq withdrawal, and some have fatigue during Pristiq withdrawal. How to Taper Psychoactive Medication gives the solutions.
Pristiq withdrawal – Nausea during Pristiq withdrawal is all too common. It does not need to happen. Read How to Taper Psychoactive Medication for the solution.
Pristiq withdrawal – Do you want a list of virtually all Pristiq withdrawal side effects? Not only does How to Taper Psychoactive Medication give the Pristiq withdrawal side effects but offers solutions.
Pristiq withdrawal – Brain zaps caused by Pristiq withdrawal can be eliminated quickly. Yes, the solution is in How to Taper Psychotropic Medication.
If you are experiencing brain zaps, electrical jolts in the head, click here
Pristiq (desvenlafaxine) is a prescription medication used to treat major depressive disorder (MDD) in adults. It belongs to the class of drugs known as serotonin-norepinephrine reuptake inhibitors (SNRIs). Pristiq works by increasing the levels of certain chemicals in the brain that help regulate mood, such as serotonin and norepinephrine.
Like all medications, Pristiq can cause side effects. One of the most common side effects of Pristiq is withdrawal symptoms that occur when the medication is stopped abruptly. Withdrawal symptoms can also occur when a patient misses a dose of Pristiq.
The severity of Pristiq withdrawal symptoms can vary from person to person. Some individuals may experience mild symptoms, while others may experience severe symptoms that can interfere with their daily activities.
Common Pristiq withdrawal symptoms include:
DizzinessNauseaHeadachesFatigueInsomniaIrritabilityAnxietySweatingTingling or numbness in the hands or feet.
Flu-like symptoms, such as chills and body ache In addition to these common symptoms, some individuals may experience more severe withdrawal symptoms, such as:
Suicidal thoughts or behavior
Mania or hypomania (symptoms of bipolar disorder)
Seizures
Hallucinations
Delirium
If you experience any of these severe symptoms, seek immediate medical attention.
Pristiq withdrawal can be challenging, but there are steps you can take to minimize the severity of symptoms. It is important to work with your healthcare provider to develop a plan for tapering off Pristiq slowly. Tapering involves gradually reducing the dosage of Pristiq over a period of several weeks or months, which allows your body to adjust to the changes in the medication.
The JNK gene, also known as MAPK8, encodes for the JNK protein, which belongs to the mitogen-activated protein kinase (MAPK) family. JNK is activated in response to various stress stimuli, such as oxidative stress, inflammation, and DNA damage. Once activated, JNK triggers a signaling cascade that leads to the activation of transcription factors, which regulate the expression of genes involved in cell proliferation, differentiation, and apoptosis.
Recent studies have shown that Pristiq can modulate the activity of the JNK pathway. In particular, Pristiq has been shown to inhibit the activity of JNK3, one of the three isoforms of JNK. This inhibition leads to a reduction in the activation of transcription factors that are downstream of JNK, such as c-Jun and ATF-2. As a result, the expression of genes involved in inflammation and cell death is reduced.
In addition to its effects on the JNK pathway, Pristiq also modulates other signaling pathways involved in the pathophysiology of depression and anxiety. For example, Pristiq inhibits the reuptake of both serotonin and norepinephrine, which are neurotransmitters involved in the regulation of mood, sleep, and appetite. By increasing the availability of these neurotransmitters in the brain, Pristiq can improve mood, reduce anxiety, and alleviate other symptoms of depression and anxiety disorders.
The exact mechanisms by which Pristiq modulates the JNK pathway are still under investigation. However, it is thought that Pristiq may act through the inhibition of the activity of certain enzymes, such as glycogen synthase kinase-3β (GSK-3β), that are involved in the regulation of JNK activity. By inhibiting GSK-3β, Pristiq may indirectly inhibit the activity of JNK, leading to a reduction in the expression of genes involved in inflammation and cell death.
In conclusion, Pristiq is a medication that has been shown to modulate the activity of the JNK pathway, a signaling cascade involved in inflammation and cell death. By inhibiting the activity of JNK3, Pristiq can reduce the activation of transcription factors that regulate the expression of genes involved in these processes. This mechanism of action, combined with Pristiq’s effects on other signaling pathways involved in the pathophysiology of depression and anxiety, makes Pristiq an effective treatment option for these conditions. However, further research is needed to fully understand the exact mechanisms by which Pristiq modulates the JNK pathway and to identify potential therapeutic targets for the treatment of depression and anxiety disorders.
Pristiq and Obesity
Obesity is a growing problem worldwide and is associated with a variety of health problems, such as cardiovascular disease, diabetes, and certain types of cancer. There are many factors that can contribute to the development of obesity, including genetics, lifestyle, and environmental factors. Medications can also contribute to weight gain, and Pristiq is one such medication.
Studies have shown that Pristiq can cause weight gain in some individuals. In one study, patients taking Pristiq for 24 weeks gained an average of 1.1 kilograms (2.4 pounds) of weight, while those taking a placebo gained an average of 0.1 kilograms (0.2 pounds). Another study found that patients taking Pristiq for 12 weeks gained an average of 1.5 kilograms (3.3 pounds) of weight, compared to an average weight loss of 0.3 kilograms (0.7 pounds) in those taking a placebo.
The exact mechanism by which Pristiq causes weight gain is not fully understood. However, it is thought to be related to its effects on certain neurotransmitters in the brain. Serotonin and norepinephrine are two neurotransmitters that are involved in the regulation of appetite and metabolism. By increasing the availability of these neurotransmitters, Pristiq may lead to an increase in appetite and a decrease in metabolism, which can contribute to weight gain.
While weight gain can be a concern for individuals taking Pristiq, it is important to note that not everyone who takes the medication will experience this side effect. Additionally, the amount of weight gain can vary from person to person. For some individuals, the weight gain may be minimal and not a cause for concern, while for others, it may be more significant and require intervention.
Metabolic Disorders>Pristiq and metabolic disorder, on the other hand, refers to a cluster of conditions that affect the body’s ability to metabolize nutrients properly. These conditions include obesity, insulin resistance, high blood pressure, and high cholesterol levels. Metabolic disorders can increase the risk of developing type 2 diabetes, cardiovascular disease, and other health problems.
Recent studies have suggested a potential link between Pristiq use and metabolic disorders. In particular, research has shown that Pristiq can cause weight gain, which is a known risk factor for metabolic disorders.
1. NAMI authors, “Desvenlafaxine (Pristiq)” National Alliance on Mental Illness [online publication] December 2018 [cited 2022 July 27]
2. Zhang, G., Gao, Z., Guan, S. et al. “Upregulation of excitatory neurons and downregulation of inhibitory neurons in barrel cortex are associated with loss of whisker inputs.” Mol Brain6,&2 (2013). https://doi.org/10.1186/1756-6606-6-2 [cited 2022 July 27]
3. Guzman F MD “Venlafaxine and Desvenlafaxine: Differences and Similarities” Psychopharmacology Institute, 2019 Jun 27 [cited 2022 July 27]
4. FDA drug label Pristiq (desvenlafaxine) 2008 [cited 2022 July 27]
5. Papp A, Onton JA. Brain Zaps: An Underappreciated Symptom of Antidepressant Discontinuation. Prim Care Companion CNS Disord. 2018 Dec 20;20(6):18m02311. doi: 10.4088/PCC.18m02311. PMID: 30605268. [cited 2022 July 27]
6. Wong J, Motulsky A, Abrahamowicz M, Eguale T, Buckeridge DL, Tamblyn R. Off-label indications for antidepressants in primary care: descriptive study of prescriptions from an indication based electronic prescribing system. BMJ. 2017;356:j603. Published 2017 Feb 21. doi:10.1136/bmj.j603 [cited 2022 July 27]
7. Rosenbaum JF, Zajecka J. Clinical management of antidepressant discontinuation. J Clin Psychiatry. 1997;58 Suppl 7:37-40. PMID: 9219493. [cited 2022 July 27]
8. Henssler J, Heinz A, Brandt L, Bschor T. Antidepressant Withdrawal and Rebound Phenomena. Dtsch Arztebl Int. 2019;116(20):355-361. doi:10.3238/arztebl.2019.0355 [cited 2021 Jun 14]
9. Gabriel M, Sharma V. Antidepressant discontinuation syndrome. CMAJ. 2017;189(21):E747. doi:10.1503/cmaj.160991 [cited 2022 July 27]
10. Berber MJ. FINISH: remembering the discontinuation syndrome. Flu-like symptoms, Insomnia, Nausea, Imbalance, Sensory disturbances, and Hyperarousal (anxiety/agitation). J Clin Psychiatry. 1998 May;59(5):255. PMID: 9632038. [cited 2022 July 27]
11. Dilsaver SC, Greden JF. Antidepressant withdrawal phenomena. Biol Psychiatry. 1984 Feb;19(2):237-56. PMID: 6324897. [cited 2022 July 27]
12. Dilsaver SC, Kronfol Z, Sackellares JC, Greden JF. Antidepressant withdrawal syndromes: evidence supporting the cholinergic overdrive hypothesis. J Clin Psychopharmacol. 1983 Jun;3(3):157-64. PMID: 6348107. [cited 2021 Jun 14]
13. Wolfe RM. Antidepressant withdrawal reactions. Am Fam Physician. 1997 Aug;56(2):455-62. Erratum in: Am Fam Physician 1998 Feb 15;57(4):646. PMID: 9262526. [cited 2022 July 27]
14. Howland RH. Potential adverse effects of discontinuing psychotropic drugs: part 2: antidepressant drugs. J Psychosoc Nurs Ment Health Serv. 2010 Jul;48(7):9-12. doi: 10.3928/02793695-20100527-98. Epub 2010 Jun 22. PMID: 20608581. [cited 2022 July 27]
15. Naseeruddin R, Rosani A, Marwaha R. Desvenlafaxine. [Updated 2021 Mar 13]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan. Available from: https://www.ncbi.nlm.nih.gov/books/NBK534829/ [cited 2022 July 27]
16. Skanland S, Cieslar Pobuda A, “Off-label uses of drugs for depression.” European Journal of Pharmacology Vol 865, 15 December 2019, 172732 as published in Science Direct [cited 2022 July 27]
17. Liebowitz MR, Tourian KA. Efficacy, safety, and tolerability of Desvenlafaxine 50 mg/d for the treatment of major depressive disorder:a systematic review of clinical trials. Prim Care Companion J Clin Psychiatry. 2010;12(3):PCC.09r00845. doi:10.4088/PCC.09r00845blu [cited 2022 July 27]
18. Thase ME, Fayyad R, Cheng RF, Guico-Pabia CJ, Sporn J, Boucher M, Tourian KA. Effects of desvenlafaxine on blood pressure in patients treated for major depressive disorder: a pooled analysis. Curr Med Res Opin. 2015 Apr;31(4):809-20. doi: 10.1185/03007995.2015.1020365. Epub 2015 Mar 26. PMID: 25758058. [cited 2022 July 27]